Safety Notice - ResMed Masks with Magnets
Urgent Field Safety Notice for Potential Magnetic Interference with Medical Devices
Table of Contents
ResMed - Mask Safety Notice
As of November 20th, 2023, ResMed has issued the below Urgent Field Safety Notice in regard to ResMed PAP masks with magnets that have the potential to cause magnetic interference with certain medical devices. We have transcribed the letter below into our learning portal for better searchability and readability. There is also a PDF copy available of the original letter at the bottom of this article. For more information, please visit www.resmed.com/magnetupdate

Patient Letter
Reference: MWM-2023-FSN-01
Date: 20 November 2023
Dear Patient,
ResMed is providing you with important information regarding our masks with magnets, which may interact with some implants or certain medical devices. It is important that you understand the information contained in this letter, take action if necessary, and keep it for future reference.
In response to new information from latest industry practices and global safety data, ResMed is updating the Contraindications and Warnings in our User Guides to instruct patients on the safe use of ResMed masks with magnets.
• Contraindications: conditions under which the mask is not to be used.
• Warnings: identify a possible hazard and provide information on how to safely use the mask.
General Product Description
These masks are used to deliver air to a patient from a positive airway pressure (PAP) device.
Magnets are used in some ResMed masks to provide a simple and easy way for patients to attach and detach the headgear to the mask frame when regularly fitting the mask for use. This can be notably beneficial to patients with disabilities, including those facing dexterity or vision impairment.
Which masks are affected?
The following ResMed masks contain magnets, please note that mask availability may differ in each country. If you do not use one of the masks identified below, this letter does not apply to you.
| Mask Type | Affected Masks |
| Full Face Mask | AirFit F20, AirFit F20 for Her, AirTouch F20, AirTouch F20 for Her, AirFit F30, AirFit F30i |
| Nasal Mask | AirFit N10, AirFit N10 for Her, AirFit N20, AirFit N20 for Her, AirTouch N20, AirTouch N20 for Her |
| Non-Vented Mask | AirFit F20 NV |
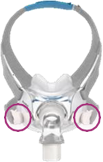
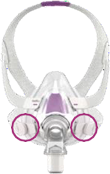
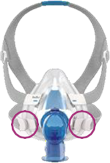
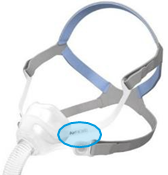
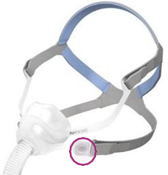
See Appendix A for more information on identifying your ResMed mask and the specific location of the magnets.
What has changed?
ResMed is updating the Contraindications and Warning relating to magnetic interference, in its User Guides for all masks with magnets.
Updated Contraindications
|
Masks with magnetic components are contraindicated for use by patients where they, or anyone in close physical contact while using the mask, have the following:
|
Updated Warning
|
Keep the mask magnets at a safe distance of at least 6 inches (150 mm) away from implants or medical devices that may be adversely affected by magnetic interference. This warning applies to you or anyone in close physical contact with your mask. The magnets are in the frame and lower headgear clips, with a magnetic field strength of up to 400mT. When worn, they connect to secure the mask but may inadvertently detach while asleep.
|
Patient Safety Information
ResMed’s masks with magnets are safe when used in accordance with the updated Instructions for Use including the Contraindications and Warnings.
When a magnet comes close to certain medical implants/devices (less than 6 inches / 150mm) there is potential for magnetic interference to change:
- the function of an implant/medical device
- the position of implants made of ferromagnetic material
In rare circumstances, this can lead to serious injury or death.
Contraindicated patients can avoid the potential harm from magnetic interference by using an alternative mask without magnets. All other patients using ResMed masks with magnets can continue to use the mask following the updated Instructions for Use.
From 2014 to November 2023, ResMed has sold tens of millions of masks with magnets globally. In this time, ResMed has submitted five (5) reports of serious harm (medical intervention /hospitalization) that were potentially related to magnetic interference with an implanted device (including implantable cardioverter defibrillators (ICD) and cerebrospinal fluid (CSF) shunts) to relevant Regulatory Authorities. No permanent injuries or deaths have been reported.
Actions to be taken by you
Contraindicated patients using a ResMed mask with magnets
If you, or anyone in close physical contact while using the mask (e.g. bed partner), have an active medical implant or metallic implanted object that is identified within the Contraindications above:
- Replace your mask containing magnets with an alternative mask without magnets in a timely manner. Contact your mask provider for alternative mask options.
Note, ResMed is making our masks without magnets available to your mask provider for replacement. - If an alternative mask is not available, consult your physician/doctor for appropriate next steps regarding your therapy.
- Dispose of the mask with magnets after you receive an alternative mask.
Note, not all models or variants of medical devices listed in the contraindications are affected by external magnetic fields. If you are not sure whether a medical device/implant falls under the Contraindications, or you require additional information on the potential adverse effects of magnetic fields for your particular device, please contact your physician/doctor.
All other patients using a ResMed mask with magnets
If you are NOT contraindicated from using a ResMed mask with magnets, you may continue to use the mask, ensuring you follow all user instructions, including the updates described in this letter.
- Importantly, ResMed masks with magnets are to be kept at least 6 inches (150mm) away from implants or medical devices that may be adversely affected by magnetic interference, as described in the updated Warning.
For more information regarding these changes please visit www.resmed.com/magnetupdate.
Reporting an Adverse Event
If you have experienced an adverse event related to the use of ResMed masks with magnets, please visit www.resmed.com/contact or contact your mask provider. Alternatively, adverse events experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, or by regular mail or by fax. 1- 800-332-1088 to request a form.
Manufacturer
ResMed Pty Ltd
1 Elizabeth Macarthur Drive Bella Vista 2153
Australia
We appreciate your support and consider this action necessary to ensure that all patients are aware of these updates. If you have any questions or concerns or would like more information, please contact your mask provider or physician/doctor.
Sincerely,

Dawn Y. Haake
Chief Quality Officer
Appendix A - ResMed Masks with Magnets
Note, product availability may differ in each country.
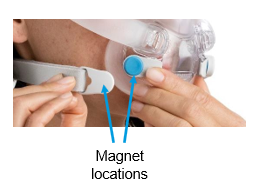
Products Affected |
Location of Model Name |
Location of Magnets |
 |
 |
 |
 |
 |
 |
    |
  |
  |
    |
 |
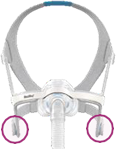 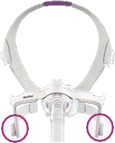 |
 |
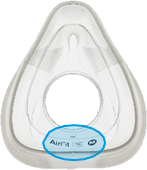 |
 |
  |
 |
 |
PDF Download